Clinical Pipeline
Qurgen is investing in scientific and technical excellence for developing a pipeline of innovative medicines that combat cancer, heart failure, diabetes, eye diseases and anti-aging.

On December 7, 2022, the US FDA granted a first-in-human Phase I clinical trial IND approval for our leading anti-cancer transcription factor drug, “SON-DP” to treat late-stage solid tumors with the focus on breast, ovarian, pancreatic and colorectal cancers (IND Number: 152226, ClinicalTrials.gov ID: NCT05989724). We had engaged with the following 5 hospitals for this clinical trial in US for Phase Ia dose escalation stage:
- MD Anderson Cancer Center
- Henry Ford Health System
- Carolina BioOncology Institute (CBI)
- Banner MD Anderson at Banner Gateway Medical Center
- Stephenson Cancer Center, University of Oklahoma
Qurgen has also obtained IRB approvals for this Phase I clinical trial of SON-DP. The first patient has undergone treatment in September of 2023. Up to August of 2024, our clinical data demonstrated the safety of SON-DP drug and also displayed initial efficacy data of tumor shrinkage and disappearance of smaller metastatic lessons in late-stage solid tumor patients at the mid-dose levels of SON-DP drug.
Clinical Pipeline and Development Plan of TF protein drugs
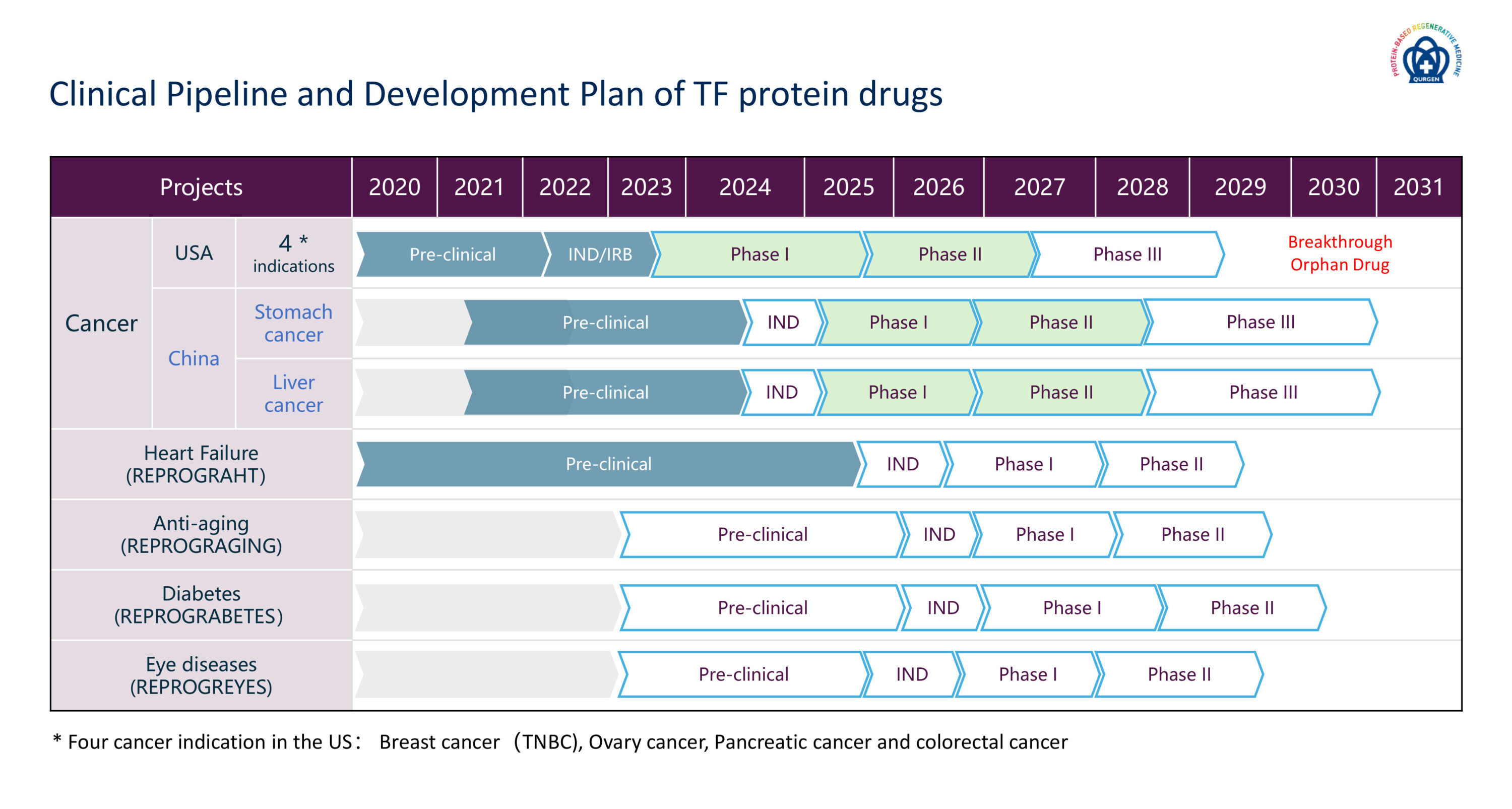
In addition to our anti-cancer drug, SON-DP, which is at a Phase I clinical trial stage in the US and soon in China with the focuses on breast, ovary, pancreatic and colorectal cancers in the US and liver and stomach cancers in China, Qurgen has also developed several transcription factor drugs for the treatment of heart failure, diabetes, eye disease (eye drops) and anti-aging. Qurgen anticipates to applying a first-in-human Phase I clinical trial in 2025 for its anti-heart failure drug, REPROGRAHT and anti-eye disease eye drop, REPROGREYES.

