- Qurgen has developed several breakthrough transcription factor (TF) protein drugs
- Qurgen has developed the 2nd generation of safe protein-induced in vivo disease tissue reprogramming technology
technology platforms
QQ-Protein Delivery Technology Platform
QQ-reagent is a polymer-based protein delivery vehicle that non-covalently associates with the protein surface and facilitate protein entry into cells. This unique delivery system, known as QQ-protein delivery, has the target capability to deliver intracellular proteins to specific cell organelle with greater than 95% protein delivery efficiency. Furthermore, targeted delivery of proteins into disease or injured or degenerated tissues can be achieved by this protein delivery platform through systemic administration of protein drugs with minimal off-target effect. Leveraging this technology, we are developing innovative medical solutions using protein drugs to reverse the course of multiple debilitating diseases including cancer, heart failure, diabetes, eye diseases and anti-aging, ultimately improving human health and longevity.
QQ-Protein Induced Pluripotent Stem Cell Technology Platform
The KEY feature of this novel QQ-piPSC technology is its remarkable greater than 90% piPSC conversion efficiency from human somatic cells or human disease cells including cancer cells. This overcomes the challenges associated with low conversion efficiency of current existing iPS technologies. Using a whole dish expansion method to cover entire starting disease cell populations, this QQ-piPSC technology is critical for drug screening and disease modeling with the minimal bias. We can now efficiently generate piPSCs from the entire starting cell population, having immense impacts for use in regenerative medicine and drug development.
Protein Induced In Vivo Tissue Reprogramming Technology Platform
Unlike the in vitro cell reprogramming techniques, this breakthrough technology allows for safe and efficient in vivo reprogramming disease cells within diseased tissue by systemic injections of QQ-reprogramming proteins. It generates transient stem cells in situ, such as transient iPSCs or transient adult stem cells, within disease tissue. The newly generated transient stem cells quickly differentiate into younger healthy tissue cells under the differentiating resident tissue environment, thus does not pose the risk of tumor or teratoma formation. This technology allows the previously undruggable transcription factor proteins to become powerful drugs, significantly accelerating bench-to-bed clinical translations of stem cell technologies in protein based regenerative medicine to treat major human disease, tissue repair and regeneration as well as reverse aging.
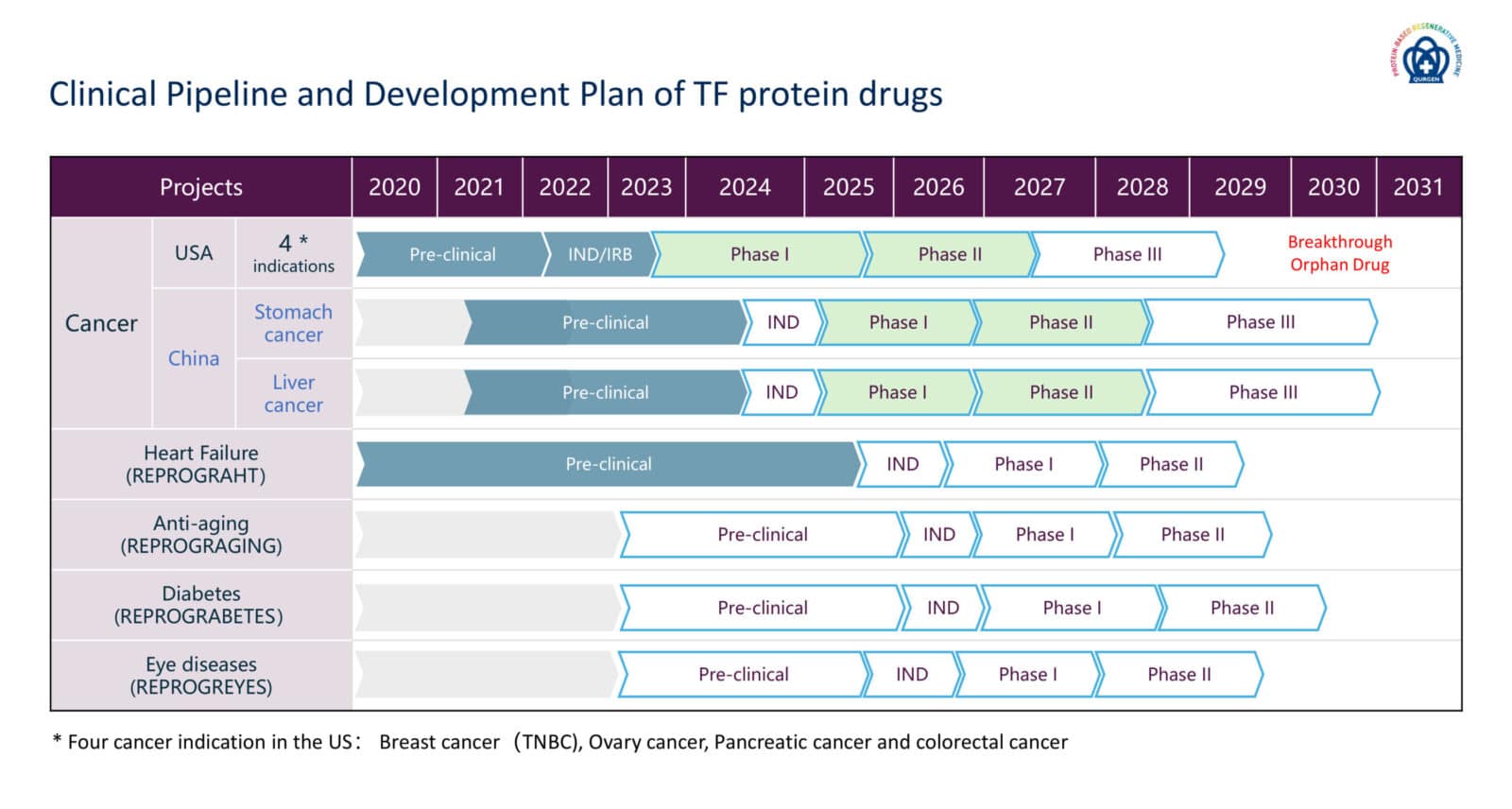
Clinical Pipeline
Qurgen is investing in scientific and technical excellence for developing and introducing a clinical pipeline of innovative medicines that combat cancer, heart failure, diabetes, eye disease and anti-aging.
On December 7, 2022, the FDA granted a first-in-human Phase I clinical trial IND approval for our leading anti-cancer transcription factor protein drug, “SON-DP” to treat late-stage solid tumors with the focus on breast cancers, ovarian cancer, pancreatic cancer and colorectal cancers (IND Number: 152226, ClinicalTrials.gov ID: NCT05989724). We have engaged with the following 5 hospitals at the Phase Ia dose escalation stage:
- MD Anderson Cancer Center
- Henry Ford Health System
- Carolina BioOncology Institute (CBI)
- Banner MD Anderson at Banner Gateway Medical Center
- Stephenson Cancer Center, University of Oklahoma
Qurgen has also obtained IRB approvals for this Phase I clinical trial of SON-DP. The first patient had undergone treatment in September of 2023. Upon to August of 2024, our clinical data demonstrated the safety of SON-DP drug and displayed initial efficacy of tumor shrinkage and disappearance of smaller metastatic lessons of late-stage solid tumor patients at the mid-dose levels of SON-DP drug.

Applications
These core technologies provide brand-new therapeutic program in treating cancer, heart disease, ophthalmology and other serious diseases, and have wide application prospect in biomedical field.
- Cancer Treatment
- Cardiac Disease
- Anti-aging and Longevity
- Other Serious Diseases
Qurgen is a clinical stage biotechnology company located in Michigan that was established in 2012. Its founders developed innovative technology platforms that serve as the basis of the company’s operations.

